Modification Kinetics of MgO–Al2O3 Inclusions by Ca-treatment |
\\ Статьи
Shufeng Yang
State Key Laboratory of Advanced Metallurgy
University of Science and Technology Beijing, Beijing, China
School of Ecological and Metallurgical Engineering
University of Science and Technology Beijing, Beijing, China
Jingshe Li
State Key Laboratory of Advanced Metallurgy
University of Science and Technology Beijing, Beijing, China
School of Ecological and Metallurgical Engineering
University of Science and Technology Beijing, Beijing, China
Xiangzhou Gao
State Key Laboratory of Advanced Metallurgy
University of Science and Technology Beijing, Beijing, China
School of Ecological and Metallurgical Engineering
University of Science and Technology Beijing, Beijing, China
Yu Ma
State Key Laboratory of Advanced Metallurgy
University of Science and Technology Beijing, Beijing, China
School of Ecological and Metallurgical Engineering
University of Science and Technology Beijing, Beijing, China
Modification Kinetics of MgO–Al2O3 Inclusions by Ca-treatment
Abstract: In this paper, high-temperature experiments were first carried out to study the modification kinetics of MgO–Al2O3 inclusions by Ca-treatment, and then a kinetic model for modification of MgO–Al2O3 inclusions by Ca-treatment was developed. The results predicted by the kinetic model agree well with the experimental results, and both show that the diffusion of Mg replaced by Ca in the inclusions is the rate-controlling step and full modification of MgO–Al2O3 inclusions requires a long time. For example, to fully modify a 12 μm inclusion, at least 20 min is required after Ca-treatment. In practice, to fully modified MgO–Al2O3 Ca-treatment should be carried out as far as possible in advance.
Keywords: MgO–Al2O3 inclusions, modification, Ca-treatment, kinetics
1. Introduction
MgO–Al2O3 spinel inclusions are unfavorable for both the quality of products and the castability of the steel because of their high melting point and high hardness.[1] To reduce the negative effects of MgO–Al2O3 spinel inclusions, it is important to modify the inclusions into liquid inclusions in the molten steel. Many studies[2–6] have investigated the modification of MgO–Al2O3 inclusions by Ca-treatment. Itoh et al.[2] found that even a very small amount of Ca in the steel can significantly decrease the stability of spinel inclusions and dramatically increase the stability of liquid MgO–Al2O3 inclusions. Young et al.[3] studied the formation mechanism of liquid calcium alumina inclusions originating from MgO–Al2O3 spinel materials, and found that spinels reacted with the dissolved Ca, forming a liquid calcium aluminate phase. Pistorius et al.[4] suggested that MgO could substantially contribute to liquefy inclusions by calcium treatment, so less calcium was needed when MgO was present in the starting inclusions. They concluded that calcium treatment can successfully modify spinel inclusions to mixed alumina–lime–magnesia inclusions and they change from irregular to globular in shape.
However, Yang et al.[7] found that many MgO–Al2O3–CaO inclusions have a two-layer structure after Ca-treatment: an outer CaO–Al2O3 layer and an Al2O3 core. YoungJo [3] found that even after 60 min of calcium treatment refining, there was still pure MgO–Al2O3 component inside the resulting MgO–Al2O3–CaO inclusion, indicating that 60min calcium treatment does not fully modify the MgO–Al2O3 inclusion into uniform CaO–MgO–Al2O3 or CaO–Al2O3 inclusions for long time calcium treatment. Therefore, the kinetic conditions are one of the key factors to determine whether the modification of MgO–Al2O3 spinel inclusions by Ca-treatment is effective.
In this study, modification of MgO–Al2O3 spinel inclusions by Ca-treatment was first investigated by laboratory experiments. Then, a kinetic model for the modification of MgO–Al2O3 inclusions by Ca-treatment was developed, and the results of model predictions and laboratory experiments are compared and the rate-controlling step for Ca-treatment is discussed.
2. Kinetic Experiments of Ca-treatment
2.1 Methodology
The description of the equipment used to carry out the experiments for modification of MgO–Al2O3 inclusions by Ca-treatment can be found in this literature.[7] The experimental steel is 30CrMo, with a composition (in wt%) of C 0.30, Si 0.22, Mn 0.52, P 0.015, S 0.007, Mo 0.10, V 0.12, Cu 0.07, and Cr 0.94. The experimental procedure is as follows. A total of 390 g of 30CrMo steel was melted in a crucible at a temperature of 1600 °C for homogenization. 0.3 g of aluminum wire was then added to the melt for . After about 5 min, 4 g of Mg was added into the melt. Ca-treatment using 4 g calcium silicide addition in powder form was performed about 10 min later. Liquid steel samples were taken by quartz tubes at various times after Ca-treatment: 1, 3, 5, 7, 12, 20, and 30 min. Some of the samples were used to analyze the composition of the steel and the others were used for the observation of inclusions.
The chemical compositions of the steel samples were analyzed by inductively coupled plasma atomic emission spectroscopy (ICP–AES) and the inclusions were analyzed by scanning electron microscopy coupled with energy-dispersive X-ray spectroscopy (SEM–EDX) and optical microscopy. For each sample, the compositions and morphologies of 40 inclusions were analyzed by SEM–EDX and the sizes of 1000 inclusions were observed by optical microscope.
2.2 Variation of Inclusion Composition
Figure 1 shows the variation of the average composition of the inclusions. After Ca-treatment, the average content of CaO sharply increases in the first 7 min, and then gradually increases from 7–30 min. After about 20 min, the value changes little and remains at about 20%. The MgO and Al2O3 contents in the inclusions rapidly decrease after Ca-treatment.
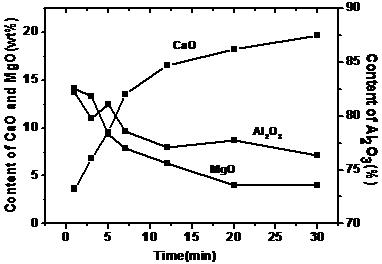
Figure 1. Variation of inclusion composition after Ca-treatment.
3. Kinetic Model of Ca-treatment for MgO–Al2O3 Inclusions
As discussed above, deoxidation by Al generates a mass of Al2O3 inclusions that will react with Mg existing in the melts. Thus, the inclusion components change from Al2O3 to MgO–Al2O3. The Mg in the melts is artificially added, although in practice MgO in the slag or ladle lining can be reduced by Al and the reaction product Mg enters into the molten steel. Calcium treatment can modify irregular-shaped spinel inclusions into globular CaO–Al2O3–MgO inclusions. The modification mechanism is (1) diffusion of the dissolved Ca in the boundary layer, (2) diffusion of Ca in the CaO–Al2O3–MgO inclusions, (3) reaction between Ca and MgO–Al2O3 at the interface, (4) diffusion of the dissolved Mg in the inclusions, and (5) diffusion of the dissolved Mg in the boundary layer.
The rate of chemical reaction occurring at the interface is much faster than the transfer rate of element diffusion in the molten steel or in the inclusions. Thus, the chemical reaction cannot be the rate-controlling step during Ca-treatment. In the following discussion, two cases were analyzed for the kinetics between the molten steel and inclusions. The first case is when the diffusion of Ca or Mg in the boundary layer is the rate-controlling step, and the second is when the diffusion of Ca or Mg in the inclusions is the rate-controlling step. This assumes that Ca first reacts with MgO–Al2O3 inclusions at the external surface, and a product layer of MgO–Al2O3–CaO inclusions forms. For simplification, in this reaction model the inclusions are treated as globular-shaped and the radius does not change with time.
The MgO in the MgO–Al2O3 inclusions is reduced by Ca during Ca-treatment, which will result in an decrease of MgO content and a increase of CaO in the inclusions. The content of MgO in spinel inclusions is 38%, so the saturation content of CaO in the inclusions after modifying by Ca-treatment should be 35.4%. Experimentally, the content of MgO in inclusions cannot be saturated, and Ca cannot decrease the total amount of MgO in MgO–Al2O3. Therefore, the actual CaO content after Ca-treatment is less than 35.4%. In this article, the saturation content of MgO in the inclusion was set at 20%, because when Ca-treatment reaches the equilibrium state in kinetic experiments the average content of CaO in the inclusion is close to 20%.
3.1 Diffusion of Ca or Mg in the Inclusion Layer as the Rate-Controlling Step
The kinetic equations and the method of calculation are completely different when treating the diffusion of Ca and Mg in the inclusion as the rate-controlling step separately, which is due to the different diffusion coefficients of Ca and Mg in MgO–Al2O3–CaO inclusions. Here, only the diffusion of Ca as the rate-controlling step will be discussed in detail. For the case of Mg, we will only show the calculated results.
Figure 2 distribution of the Ca content when the rate-controlling step is the diffusion of Ca within the MgO–Al2O3–CaO inclusions. Sp represents the MgOAl2O3 inclusion and SpC is the MgO–Al2O3–CaO inclusions. [Ca] represents the calcium content in the melt, which in this case is constant, [CaO] is the calcium oxide concentration in the MgO–Al2O3–CaO inclusions, [Ca]* is the calcium concentration at the reaction interface, ro is the radius of the inclusion, which is assumed to be constant, and r is the radius of MgO–Al2O3 inside the inclusions, which will decrease with time. The thickness of the MgO–Al2O3–CaO inclusion layer can be expressed as ro−r, and changes with time.
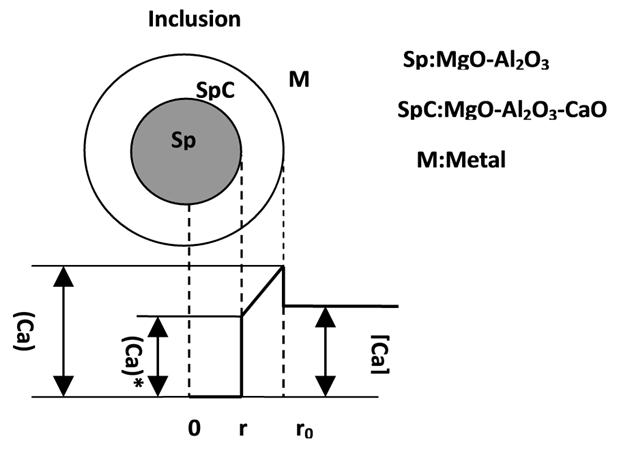
Fig. 2 Distribution of Ca content when treating the diffusion of Ca in the inclusion layer as the rate-controlling step.
The kinetic equation is built based on the relationship that the change of the CaO content in the inclusion is equal to the amount of diffusion per unit time:
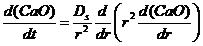 (1)
(1)
Equation (1) is a type of partial differential equation that is difficult to solve through analytical methods, and it is better to employ numerical methods to obtain the approximate solution at a certain precision. In this kinetic model, we used the finite difference method to solve the basic equation. Finite difference methods can be divided into direct difference and implicit difference methods. Starting from the differential equation, the method replaces the derivative by the difference quotient, and connects the differential equation and the solution under definite conditions into a linear system, which can be solved to obtain a numerical solution of discrete points. Therefore, the choice of space and time step is limited during the process of equation discretization. This model adopts the implicit difference method to solve the equation, and then based on the discrete equations we wrote a code to solve the equation. Treating the concentration range of CaO in the inclusion as the boundary condition, the model will calculate the time for a given size inclusion particle to grow to saturation.
The boundary conditions in this calculation are that at t=0 and r=r0 then [CaO]=20%, at t=0 and r≠r0 then [CaO]=0, and when t≠0 then [CaO]=0 at the interface between the MgO–Al2O3 and MgO–Al2O3–CaO inclusions. For the diffusion coefficient of Ca in the MgO–Al2O3–CaO inclusion, we used the diffusion coefficient of Ca in the MgO–Al2O3–CaO inclusion layer: DCa-Inc = 2.5 × 10−9 m2/s.[9] In the calculations, we chose r0=6 μm.
According to the boundary conditions and parameters, we use the computer code to carry out the calculations and obtained the results shown in Fig. 3. The content of CaO rapidly increases in the initial stage after Ca-treatment, at about 100 s the CaO content is close to saturation, and the CaO content is fully saturated at 200 s.
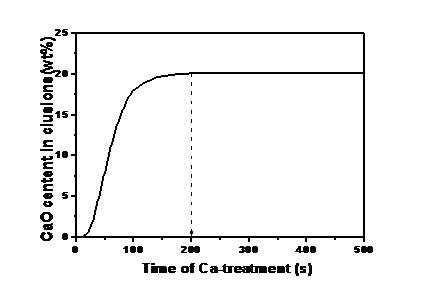
Fig. 3 Calculated result of the CaO content in an inclusion when treating the diffusion of Ca in inclusion layer as the rate-controlling step.
As mentioned above, when the rate-controlling step is the diffusion of Mg in the inclusion layer, the kinetic equation, the calculation method, and the boundary conditions are the same as when Ca is considered to be the rate-controlling step. The only difference is the diffusion coefficient. For the diffusion coefficient of Mg in the spinel inclusion, we used the diffusion coefficient of Mg in the MgO–Al2O3–CaO inclusion layer: DMg-Inc = 3.2 × 10−13 m2/s.[9] The change of CaO content with time is shown in Figure 4, which shows that the time needed for the CaO content to become saturated in the inclusion is about 1200 s.
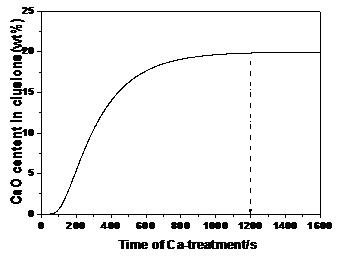
Fig. 4 Calculated result for the CaO content in inclusions with respect to time of Ca-treatment(s) when treating the diffusion of Mg in the inclusion layer as the rate-controlling step.
3.2 Diffusion of Ca or Mg in the Boundary Layer as the Rate-Controlling Step
When diffusion of Mg and Ca in the inclusion is sufficiently fast, the rate-controlling step will be the diffusion of Ca or Mg in the boundary layer during the process of Ca-treatment, and the concentration of Ca and Mg in the inclusion is assumed to be constant. The diffusion of Ca and Mg in the boundary layer and the diffusion equations of Ca and Mg are the same. Because the difference between the diffusion coefficients of Mg and Ca is small, they will be regarded as the same during the calculations. Thus, they will also have the same kinetic model. Consequently, only the diffusion of Ca in the boundary layer will be discussed.
Figure 5 shows the distribution of Ca content when the rate-controlling step is the diffusion of Ca within the boundary layer. Thus, based on the equilibrium state of [Ca] at the interface between the molten steel and the inclusions, the kinetic equation is
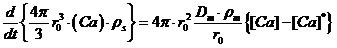 , (2)
, (2)
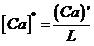 , (3)
, (3)
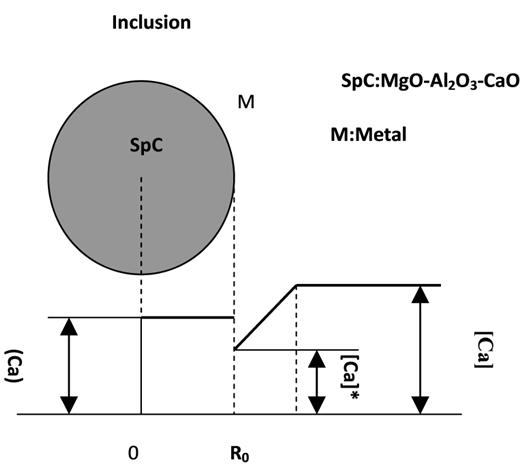
Fig. 5 Distribution of Ca content when the diffusion of Ca in the steel boundary layer is the rate-controlling step.
where (Ca) is the concentration of calcium in the inclusion, [CaO] is the concentration of calcium in the steel, [Ca]* is the calcium concentration close to the steel side on the reaction interface, and (Ca)′ is the calcium concentration close to the inclusion side on the reaction interface. Because the size of the inclusions is quite small ((1.0–5.0) × 10−6 μm), we assume that there is material transmission only on the liquid steel side of the interface; namely, (Ca) is equal to (Ca) ′.
L represents the equilibrium distribution ratio of Ca on the steel/inclusion interface. The Ca-treatment reaches an equilibrium after adding 0.0078% SiCa powder (Ca content = 1.87 × 10−7%), and the inclusion content is 13.6 × 10−2% in which CaO accounts for 20.9%, and thus L = 6.0 × 105. The diffusion coefficient of Ca and Mg in steel is Dm=3.5 × 10−9 m2/s.[10]
From Eqs. (2) and (3), and assuming that the content of Ca in steel is constant, (Ca) in the inclusion can be expressed as follows:
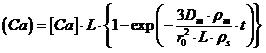 (4)
(4)
The relationship between the time of the Ca-treatment and the CaO content in the inclusion is shown in Figure 8.
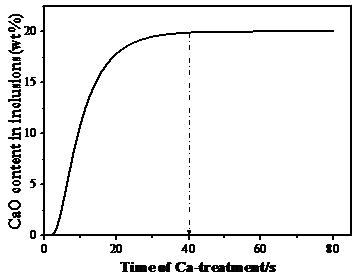
Figure 6 Content of CaO in inclusions when treating the diffusion of Ca and Mg in the boundary layer as the rate-controlling step.
Figure 6 shows that when the diffusion of Ca and Mg in the boundary layer is the rate-controlling step, CaO in the inclusion quickly becomes saturated (40 s), which indicates that Ca and Mg diffuse faster in the boundary layer of molten steel than the diffusion of Ca and Mg in the inclusion layer.
Based on the discussion above, when Ca diffusion in the inclusion is the rate-controlling step, it will take 200 s for CaO to be saturated in the inclusion, while if Mg diffusion in the inclusion is the rate-controlling step, it will take 1200 s for CaO to be saturated in the inclusion. When diffusion of Ca and Mg in the boundary layer of molten steel is the rate-controlling step, it will take 40 s for CaO in the inclusion to become saturated. For the Ca-treatment, the diffusion of Mg in the inclusion is the slowest, thus diffusion of Mg in the inclusions is the rate-controlling step.
Figure 7 shows the comparison of the experimental and prediction results for diffusion of Mg in the inclusion. The results are in good agreement, which means that the diffusion of Mg in the inclusion is the rate-controlling step in Ca-treatment, and proves the reliability of the model. However, in Figure 7 at the earlier stage of Ca-treatment there is a significant difference between the experimental and predicted results, with the experimental results being greater than the predicted results. This may be due to the non-continuity of sampling in the earlier stage and taking too many samples, because in the sampling process with quartz tubes this may cause stirring of the molten steel and speed up the reaction. In addition, it takes longer for saturation of CaO in the experimental results than in the predicted results, which may be caused by assuming that diffusion of Ca in the inclusion is too fast in the predictions; even though Ca diffuses faster than Mg in the inclusions, their diffusion rates only differ by one order of magnitude.
Both the experimental and predicted results show that 20 min of Ca-treatment is required for modification of MgO–Al2O3, although this time can be shortened by gas blowing stirring in the real manufacturing process. However, it is still recommended that Ca-treatment is performed during the refining process and not after.
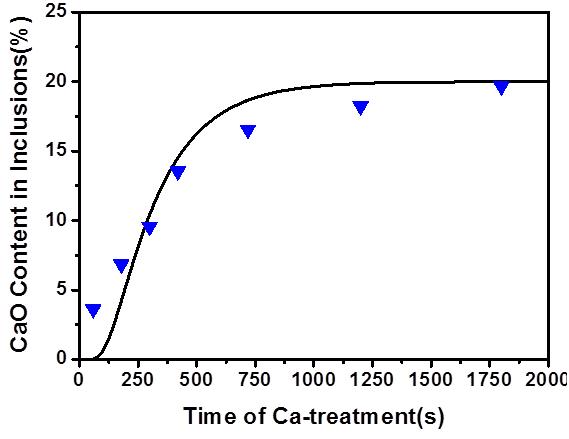
Figure 7 Comparison of the results of the model and experiment of the CaO content in inclusions with Ca-treatment time.
4. Conclusions
1) A kinetic model is developed based on the experiment results to predict the time of modification of MgO–Al2O3 inclusions. The results predicted by the kinetic model and those obtained by experiments are in good agreement.
2) Ca and Mg diffuse faster in the boundary layer of molten steel than in the inclusions, and Ca diffuses faster in the inclusions than Mg. Thus, diffusion of Mg in the inclusions is the rate-controlling step.
3) It takes considerable time for the MgO–Al2O3 inclusions to be fully modified. For a 12 μm inclusion, at least 20 min are needed after Ca-treatment. Thus, for fully modified MgO–Al2O3 in practice, Ca-treatment should be carried out as far as possible in advance.
Acknowledgments
The authors are grateful for support from the National Science Foundation China(51304016).
References
- Shufeng Yang, Jingshe Li, Lifeng Zhang. Behavior of MgO∙Al2O3 based inclusions in alloy steel during the refining process. Journal of iron and steel research international, 2010,Vol. 17(7): 1-6 .
- H. Itoh, M. Hino and S. Ban-Ya. Thermodynamics on the Formation of Spinel Nonmetallic Inclusion in Liquid Steel. Metallurgical and Materials Transactions B, 1997, vol. 28 (5), pp. 953-56.
- YoungJo, K., Fan,L.,Kazuki, M. and Du, S. Mechanism study on the formation of liquid calcium aluminate inclusion from MgO-Al2O3 spinel. Steel Research International, 2006, vol. 77 (11), pp. 785-92.
- P.C. Pistorius, P. Presoly and K.G. Tshilombo. Magnesium: Origin and role in calcium-treated inclusions. Procceding of TMS Annual Meeting and Exhibition, San Antonio, TX,2006, vol. 2, pp. 373-78.
- Shufeng Yang, Lifeng Zhang, Liyuan Sun, Jingshe Li, Kent D. Peaslee. Investigation on MgO∙Al2O3-Based Inclusions in Steels. AIST Translations, Vol.9(8), 2012, pp. 1-15
- Shufeng Yang, Jingshe Li, Zaifei Wang. Modification of MgO•Al2O3 spinel inclusions by Ca-treatment in Al-killed steel. International Journal of Minerals, Metallurgy and Materials, 2011,18(1):18-23
- Shufeng Yang, Lifeng Zhang, Jingshe Li, Kent Peaslee. Formation and Modification of MgO∙Al2O3 Based Inclusions in Alloy Steel. Metallurgical and Materials Transactions B 2012, pp.731-750
- J.H. Park, D.S. Kim and S.B. Lee. Inclusion control of ferritic stainless steel by aluminum deoxidation and calcium treatment. Metallurgical and Materials Transactions B, 2005, vol. 36 (1), pp. 67-73.
- G. Okuyama, K. Yamaguchi, S. Takeuchi and K.I. Sorimachi. Effect of slag composition on the kinetics of formation of MgO•Al2O3 inclusions in aluminum killed ferritic stainless steel. ISIJ International, 2000, vol. 40 (2), pp. 121-28.


